“if cholera should appear, you might almost expect to pump it by the gallon”
See here for details of William Harry Harris’s career and family.
Providing sufficient clean water to the inhabitants of the expanding population of Northampton proved a challenge for much of the 19th century. Various extensions and refurbishments of older schemes were undertaken from time to time. In 1836 a Water Company was established. Despite these improvements, the supply continued to struggle to meet the increasing demands on it. Additional wells were sunk across the town and pumps were installed. There were frequent questions raised about the quality of the water both in respect of hardness and cleanliness.
William Harry Harris’s profession as an analytical chemist ensured that he was commissioned from time to time to evaluate the quality of the water in Northampton, both at the request of the Town Council, the town’s Improvement Commissioners and on his own account. The results of his water testing were often published in the local press. This has a two-fold use for a historian as it indicates the quality of the water supplied and the location of the sources of the samples.
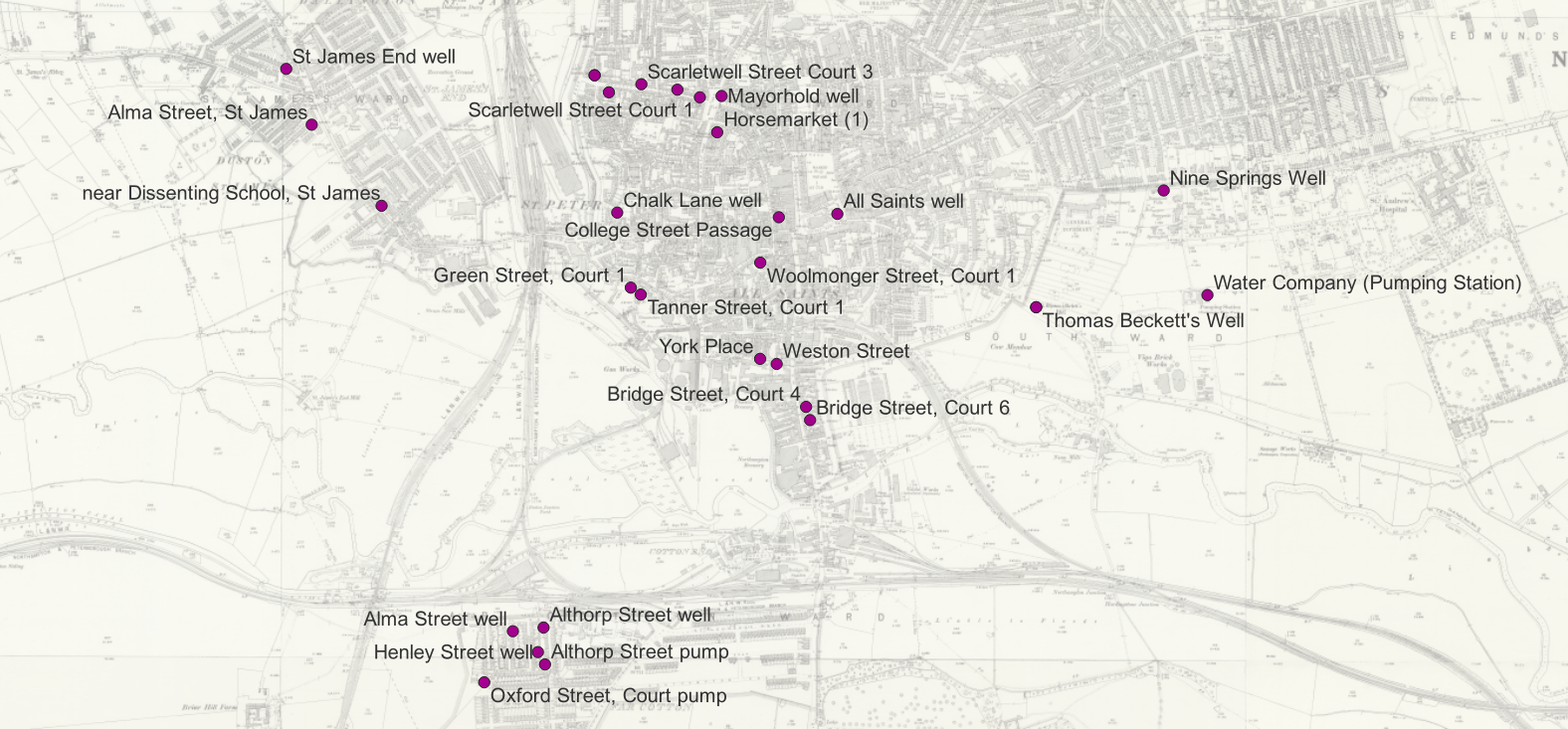
The locations of the sources of the water samples are shown on this map. Due to the limitations and availability of historic mapping of the town some wells and pumps cannot be located precisely.
The first example of this work appears in March 1860.1 At the time the majority of the town’s piped water supply was from the Water Company’s works on the Billing Road, evidently supplied by a well and springs on the site. A typical example of his report reads:
Several statements have lately been made respecting the town water, asserting that it contains a large percentage of highly injurious substances, but the result of my analysis leads me to suppose those statements were made without first submitting the water to a proper examination.
One gallon, of 70,000 grains, of the town water, at 60 deg. F. Contains:
Oxide of Iron 0.25 grain
Lime 6.38 grain
Magnesia 0.93 grainI have also had an opportunity of examining samples of water direct from the company’s deep well, bottom and top springs. In the two first I estimated, as in the town water, the quantity of iron, lime, and magnesia they contain.
It is very evident, as the deep well water is so decidedly superior to the bottom spring in quality, that if they are not always mixed in the same proportions an analysis of the water made at intervals will give a different result, which I have found to be the case.
There is nothing to complain of in the water we are at present supplied with, but if the company could obtain all they require from the deep well they would give us a water very far superior in quality to the water supplied to most other towns.
He goes on to examine the water in other significant wells in the town and finds the degrees of hardness to be:
| Degrees of hardness. | |
| Pump in College Street. Passage. | 24.2 |
| Spring in Scarletwell Street. | 28.6 |
| Thomas-a-Becket’s Well. | 24.69 |
| Nine Springs. | 28.8 |
When it is known that each of the above degrees correspond to about one grain of carbonate of lime in the gallon, the general reader will better understand the character of the springs.
I remain, sir, yours respectfully,
W. H. Harris
William Harris wrote again in the Northampton Mercury in June 1861 following the testing of several wells across the town.2
|
Source of Water |
* Degrees of Hardness |
Nitric Acid detected without evaporation, quantity not estimated |
Total solid content in the gallon. Grains |
Organic matter in the gallon Grains |
Date of Examination |
|
Well, St. James’s-End, much used |
30 |
Considerable |
… |
. |
March 1861 |
|
Well in neighbourhood of All Saints’ Church, much praised for its purity |
31.2 |
Considerable, no doubt in part derived from Church Yard |
… |
. |
“ |
|
Well on Mayorhold. |
30.5 |
Distinct trace |
… |
3.5 |
May 1861 |
|
Well in Chalk lane, much used |
36.1 |
Very considerable |
43.40 |
4.0 |
“ |
|
Tap Water |
11 |
None |
35.3 |
0.80 |
“ |
|
Well in Alma-street, Far Cotton |
27.4 |
Considerable |
. |
… |
March 1861 |
|
Well in Althorp-street, Far Cotton |
… |
Very considerable |
. |
4.0 |
May 1861 |
|
Near to the above |
… |
Very considerable |
105.5 |
4.50 |
June 1861 |
* Each degree corresponding to about one grain of carbonate of lime in the gallon.
He goes on the explain the results:
In nearly all well waters of towns, including some of average quality, nitric acid is generally to be detected, and even when existing in considerable quantity, instead of causing the water to be disagreeable, it has a contrary effect, for there are several wells in London which, although they are very highly charged with nitrates and organic matter, find much favour with the inhabitants in their vicinity; still, no person would say such waters are wholesome.
We have abundant evidence of the influence impure water possesses in pre-disposing the system to disease, and the continued use of a water from 20 degrees to 40 degrees of hardness (independently of any organic matter or substances) the result of the oxydation of organic matter it may contain should be avoided.
The reason of the water in Far Cotton varying from indifferent to bad is accounted for by the ground being low and difficult of drainage, and the wells being frequently sunk near to the cesspools. If the use of the water there should be long continued, in all probability, as the inhabitants increase and the cesspools become filled, the place will sooner or later become a hotbed for epidemic disease.
It would be an interesting consideration whether much of the illness with the poor inhabitants of the town is not dependent to a very great extent upon the water they are in the habit of drinking.
I remain, yours very respectfully,
WM. H. HARRIS.
In June 1866 he returns to the state of the well waters in the town in a lengthy letter and a summary of the state of the water in Far Cotton.3 The letter is headed with the rather grand title in capitals:
EXPERIMENTS ON THE SANITARY CHARACTERISTICS OF THE AIR AND WATERS OF NORTHAMPTON.
Sir,-Health is admitted to be largely influenced by local conditions, especially in regard to the purity of the water supply and atmosphere of a given locality. Were there any suggestions necessary to create popular interest in such a subject, the most effective one would be to direct attention to the extent to which the above-named principle may be carried.
Medical men, including those of our own town, have informed us that during the present year there is a great probability of our being visited by choleraic illness [Cholera]. During the epidemic of 1853-4, we find those districts suffered most which were supplied with impure water.
He then continues to explain his method of taking samples and the analysis and follows by detailing the results. All of the locations are in Far Cotton in the south of the town and across the River Nene.
|
|
Total solid matter in grains per gallon |
Organic and other volatile matters in grains per gallon |
Oxygen required for oxidation of organic matter per gallon |
Ammonia in grains per gallon |
Nitrites in comparative degrees Quantity not estimated |
Nitrates in comparative degrees Quantity not estimated |
Total hardness by Clarke’s scale * |
Infusoria + |
|
Pump in yard near bottom of Althorp Street |
90.4 |
5.33 |
0.12 |
0.241 |
3 |
1 |
42 |
Few. |
|
Open well in yard, Henley Street |
168.98 |
14.55 |
0.28 |
0.053 |
3 |
30 |
56.2 |
Many |
|
Pump in court, Oxford Street |
130.45 |
16.1 |
0.36 |
0.045 |
2 |
20 |
46.4 |
Few. |
|
Pump in court left-hand side Oxford Street |
58.56 |
19.64 |
0.72 |
0.21 |
30 |
15 |
27 |
Full. |
|
Pump near top of Althorp Street |
57.26 |
10.78 |
0.4 |
0.175 |
20 |
10 |
27.9 |
None. |
- If these degrees are multiplied by 2 the product will give in round numbers the ounces of soap 100 gallons of the water require for washing purposes .-
+ This examination is simply comparative, an equal quantity of the waters being taken, and the same magnifying power used.
A final analysis
|
The final instalment of William Harris’s analysis appeared in August 1866 and included details of twenty-three samples from pumps and wells across the town.4 They are summarised here:Court 1, Horseshoe Lane |
… contained the enormous quantity of two hundred and four grains in the gallon, of which twenty-two and a-half grains were organic matter. The water was very hard from the products of putrefaction, and the ammonia present, which is but converted sewage, was equal to 035 grains of carbonate of ammonia in the gallon. No comment on these results is necessary, and the use of the well should be immediately prohibited. |
|
Henley Street, Far Cotton (open well) |
… contained one hundred and sixty-nine grains of total impurity, of which fourteen and a-half grains were organic matter. It contained also thirteen grains and three-quarters of chlorine, equal to nearly twenty-three grains of common salt, showing the presence of sewage water. |
|
Oxford Street, Far Cotton (two samples) |
… gave very bad results. One contained over one hundred and thirty grains of solid impurity, and sixteen grains of organic matter, holding in solution also chlorine equal to fourteen and a half grains of salt. The other contained fifty-eight and a half grains of total impurity, and the large quantity of nineteen and a-half grains of organic matter, the chlorine present being equal to nearly thirteen grains of salt. The last water was full of animalcules5; it gave also ammonia equal to half a grain of carbonate of ammonia. |
|
|
All the waters from Far Cotton indicate the proximity of some source of contamination which is charging the wells with the most unwholesome and disgusting matters-the excretions from the bodies of the inhabitants themselves. |
|
Courts 2,3,4,5 & 6, Bridge Street |
… they are all of a most impure character, the solid impurity ranging from one hundred and three grains to one hundred and eighty-seven grains in the gallon, and the organic matter from nine to fourteen grains. The degree of hardness is excessive, varying from thirty to forty-eight degrees. This quality, instead of being due to the lime and magnesium salts existing naturally in the water, is in part caused by the presence of chloride of sodium (common salt), nitrites, and nitrates, arising from the direct infiltration of liquid sewage, and the products of its putrefaction. |
|
York-Place, Western-Street, Court 1, Green-Street Court 1, Tanner-Street |
… are of an equally objectionable character. |
|
Mayorhold (pump) |
… is an exception to the above, being of good average quality, giving fifty-six and a-half grains of solid impurity in the gallon, of which four and a.half grains are organic matter, and containing only a slight trace of either ammonia or nitric acid. |
|
Courts 1,2,3 & 5, Scarletwell Street |
… range from one hundred grains of total impurity, and two and a-half-grains of organic matter, to seventy-three and a-half-grains of total impurity, and nine and a-half grains of organic matter: the latter quantities are from the water in No. 3 Court. The fact that this water contains by far the smallest quantities of ammonia and nitric acid might lead to the false conclusion that it compared favourably with the waters from the other courts. It must be understood that the ground in the neighbourhood of the polluted well acts, until it is exhausted, as a sort of natural laboratory, in which the process of conversion is going on, from the one condition of organic matter into these less noxious products of its oxidation, so that a water containing much organic matter, and only little ammonia and nitric acid, may be the most deleterious. |
|
Scarletwell Street, Scarletwell Spring (public pump) |
… is slightly contaminated from the infiltration of surface drainage or the products of the decomposition of animal matter. … Most probably the organic impurity finds its way into the cistern or well the spring supplies. |
|
Court 1, Woolmonger Street |
… contains, in the gallon, one hundred and a-half grains of total impurity, of which fifteen and a-half grains are organic matter; there is a considerable quantity of nitrates and many animalcules, and the examination of this water indicates considerable organic contamination. |
|
Alma Street, St James |
… contained sufficient ammonia to render it very strongly alkaline, the quantity being equal to two and a-half grains of carbonate of ammonia in the gallon; there was chlorine equal to 16 grains of common salt, and also thirteen grains of organic matter. It was not ascertained if this water was applied to drinking purposes; but, if so, its use must be attended with extreme danger, for it is scarcely possible to condemn, in terms too strong, the sanitary qualities of such a water. |
|
Near the Dissenting School, St James |
… it contained eighty-one grains of solid impurity, of which over fourteen grains are organic matter. Both these wells are sinks of pollution, from whence, if cholera should appear, you might almost expect to pump it by the gallon. |
|
Town piped supply (provided by the Water Company) |
… contained forty-two grains of total impurity, of which three grains were organic matter. There was not any trace of the products of the decomposition of animal matter, and its degree of hardness was under eight degrees. The mineral residue contained a considerable quantity of soda salts and three and three-quarters grains of chlorine, both of which are present in most artesian well waters. The superiority of this water for drinking purposes, over the above, excepting the Mayorhold pump and Scarletwell spring in its normal state, is very great indeed. |
He then quotes a leader article from The Times newspaper:
“Wherever cholera appears, the utmost vigilance ought to be directed to two points-first, to the prompt disinfection of the discharges, clothes, and houses of the sick, and secondly, to the condition of the water supply. In some cases, it may be desirable to close the wells and pumps altogether, but in all cases, the water should be carefully examined.”
In conclusion, his recommendations were:
I cannot conclude without stating a powerful impression my mind has received in the course of the above examinations, and that is, of the duty of the influential men of Northampton to interest themselves in seeing the poorer class of people supplied with a good and wholesome supply of water. The first step they should take, I think, in accomplishing that purpose, is to see this class of persons in possession, where necessary, of the Company’s supply, which, both for the purposes of health and of economy, is as good as can be procured. But there is a second step also incumbent on them, and that is, to see that all the natural springs in the town are saved where preservation is possible, for good as the Company’s water is, it is most undesirable that 40,000 or 50,000 people should be obliged to depend upon one source of supply.
This seems to suggest a two-fold approach of taking the water supply into wider public ownership and investing in the existing wells and pumps to ensure an alternative source of supply.
- Northampton Mercury, 10 March 1860
- Northampton Mercury, 22 June 1861
- Northampton Mercury, 9 June 1866
- Northampton Mercury, 11 August 1866
- a microscopic animal
© Copyright : Graham Ward. All rights reserved.